
Research
The Ingram lab has several projects that examine brain circuits and synaptic plasticity associated with pain and chronic drug use. Studies mainly use whole-cell patch-clamp electrophysiology and optogenetics to isolate specific synapses within brain circuits. Understanding how drugs of abuse and pain regulate synapses will help to develop better treatments for pain and drug addiction.
Current Funded Projects
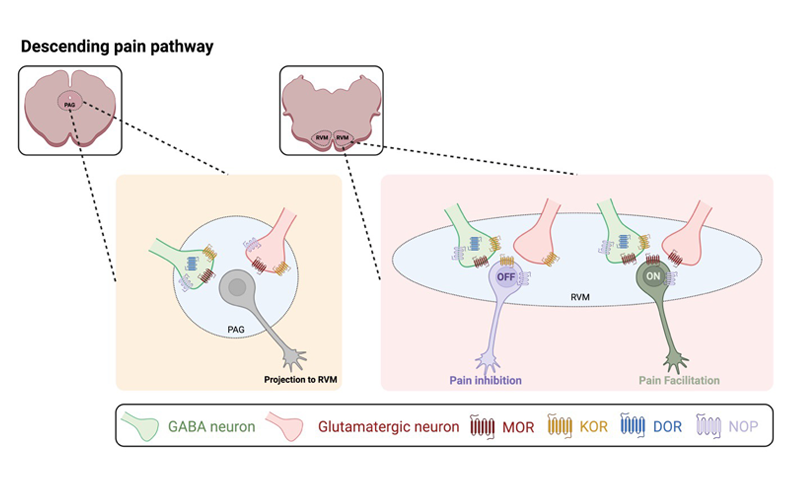
Defining the descending pain modulatory circuit (MPI-R01)
The central nervous system has an intrinsic pain modulatory system that regulates nociceptive processing through descending projections from the brainstem to the spinal cord dorsal horn. The ventrolateral periaqueductal gray (vlPAG) integrates sensory information with input from higher cortical and subcortical areas and sends projections to the rostral ventromedial medulla (RVM) that are relayed to the dorsal horn of the spinal cord. Both the vlPAG and RVM are heterogenous with respect to participating in multiple behavioral circuits. Proposed viral optogenetic strategies will map and define the vlPAG circuit that regulates RVM ON-cells involved in the facilitation of pain and elucidate underlying cellular mechanisms that shift the balance of RVM output from inhibition of pain to facilitation of pain with persistent inflammation.
A better understanding of molecular, cellular, and circuit-level mechanisms that underlie pain is essential if we are to develop better treatments. By carefully mapping the descending projections from PAG to RVM during the development of persistent inflammation, and by tying these to defined RVM outputs and behavior, we can begin to determine the interactions in this complex network and gain new insights into how pain-modulating systems are recruited and modulated in acute and chronic pain.
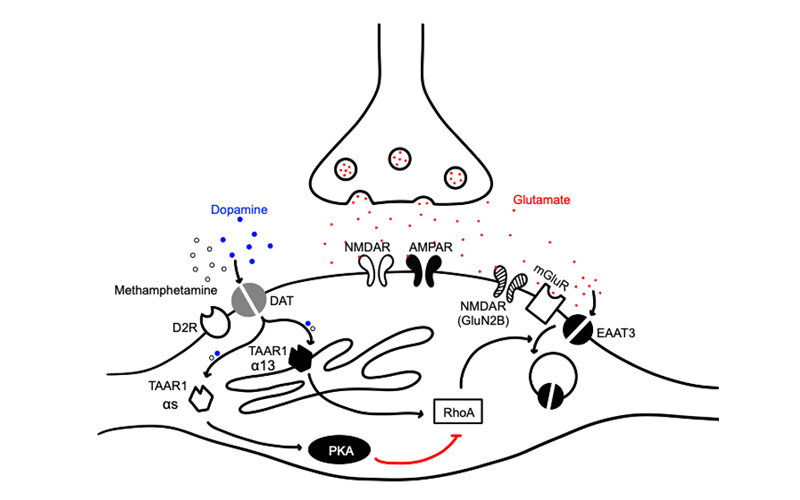
Role of lateral habenula in methamphetamine TAAR1-mediated synaptic plasticity and aversion (MPI-R01)
Considerable research has focused on drug use disorders as motivational disorders involving inherent or drug-induced reward pathway function. However, the focus of this application is on opposing aversive effects of methamphetamine (MA) that may curb its use, which have been little studied. The Richards (Phillips) laboratory identified the trace amine associated receptor 1 (TAAR1) as a critical target impacting MA-induced aversion. TAAR1 is an intracellularly located G protein-coupled receptor. MA gains access to TAAR1 only if it is transported into the cell. This occurs via extracellular membrane transporters, such as dopamine and serotonin transporters, DAT and SERT, respectively. We propose that TAAR1 activation by MA in dopamine and serotonin neurons is responsible for MA-induced aversion and that monoaminergic circuit interactions with the lateral habenula (LHb) are of particular importance. The overarching goal of the studies proposed in this application is to understand the monoaminergic neuron-LHb interactions responsible for the experience of MA-induced aversion via TAAR1 that may reduce risk for MA use. The study of mechanisms underlying sensitivity to MA-induced aversion could lead to the identification of a new class of therapeutics.
The role of delta opioid receptors in trigeminovascular pain (R01-subcontract PI)
Overuse of medications commonly prescribed for headache management can result in a contradictory increase in pain sensitivity, known as medication overuse headache. The Pradhan lab has identified two novel targets that block this medication exaggerated pain – the delta opioid receptor and the PACAP-PAC1 receptor. The aim of this proposal is to determine if these two targets commonly interact to inhibit medication overuse headache. The Ingram Lab has a subcontract to determine the underlying cellular mechanisms of PACAP and delta opioid receptors in the periaqueductal gray (PAG) and pain circuits.
Pending Projects
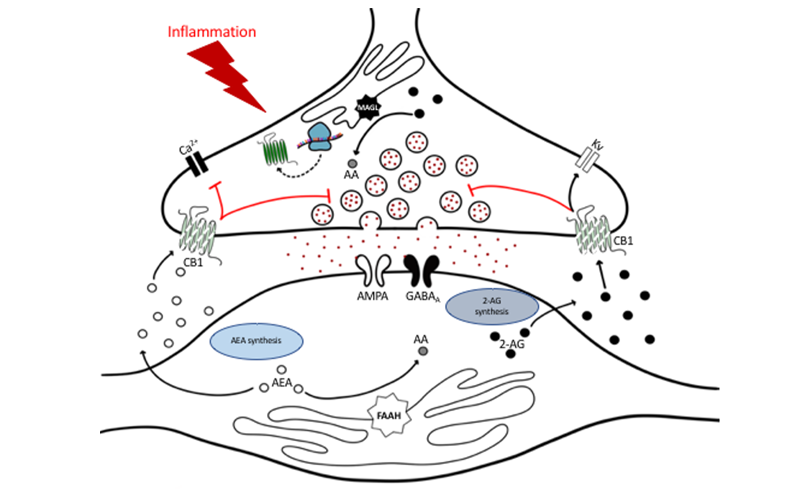
Cannabis Use Impact on Pain and Recovery Post-Surgery – The Role of the Endocannabinoid System (RM1)
The rapid rise in cannabis legalization has allowed patients to self-medicate resulting in altered states of endocannabinoid (eCB) synthesis and degradation, and receptor activation prior to seeking medical care, and the clinical consequences of an altered eCB system due to exogenous cannabinoids are unknown. We propose a comprehensive, systematic and highly integrated multidisciplinary basic and clinical science approach to characterize the a) human eCB system response to chronic pain associated with osteoarthritis, b) acute pain directly post-surgery, c) pain resolution or pain chronification in total knee arthroplasty (TKA) patients 6-months post-surgery and d) to mechanistically study changes in eCB signaling in preclinical animal models. Our goal is to understand the intersection between cannabis use and eCBs in the maintenance and resolution of chronic inflammatory pain.
Ingram Laboratory | Department of Anesthesiology | University of Colorado
Ingram Laboratory
Department of Anesthesiology
University of Colorado